Abstract
Background
VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome is an adult-onset autoinflammatory disease caused by somatic mutations in UBA1, the major E1 enzyme that initiates ubiquitylation. Patients present with cytopenias (macrocytic anemia and lymphopenia being most common), rheumatologic manifestations with elevated inflammatory markers and have increased predisposition to myelodysplastic syndrome (MDS) and plasma cell dyscrasia (PCD). However, the sequence of events, changes in blood counts and the prognostic impact, have not been fully elucidated. We carried out this study on Mayo Clinic patients with VEXAS syndrome to characterize the timeline and impact of hematologic manifestations.
Methods
After IRB approval, confirmation of VEXAS diagnosis was carried out by an inhouse PCR assay that was able to detect UBA1 variants in exons 2-3 and associated splice acceptor/donor cites. Date of symptom onset and correlating laboratory changes were retrospectively abstracted and profiled over time. Standard WHO criteria were used to define cytopenias and MDS evolution (Arber et al Blood 2016). Survival analysis was estimated by the Kaplan-Meier method.
Results
Thirty-eight patients with molecularly defined VEXAS were included in the study, with a median age of 66 years (range, 45-83). Twenty-one patients (55%) had UBA1mut p.M41T c.122T>C, 11 patients (29%) had p.M41V c.121A>G, 2 patients (5%) had p.M41L c.121A>C and 4 patients (10%) had splice site mutations (c.118-1G>C). All patients were white males. Thirteen patients (34%) had a history of tobacco use and 60% had a personal history of venous thromboembolism.
Thirty-two patients had somatic mutation testing by next-generation sequencing (NGS). Nineteen of those (59%) had pathogenic somatic mutations detected.. Most frequent somatic mutations included mutations in TET2 (n=7) with median variant allele frequency (VAF) of 6% (range, 1.2-37%), DNMT3A (n=5) with median VAF of 41.5% (range, 5-46%), and SMC3 (n=3) with median VAF of 7.5% (range, 3-77%). Seven patients (18%) met WHO criteria for MDS. All MDS patients had <5% blasts and a normal karyotype. Six of 7 assessable MDS patients had NGS data and somatic mutations were seen in 2 (33%). These included pathogenic mutations in TET2 (n=1, VAF 37%) and DNMT3A (n=1, VAF 41%).
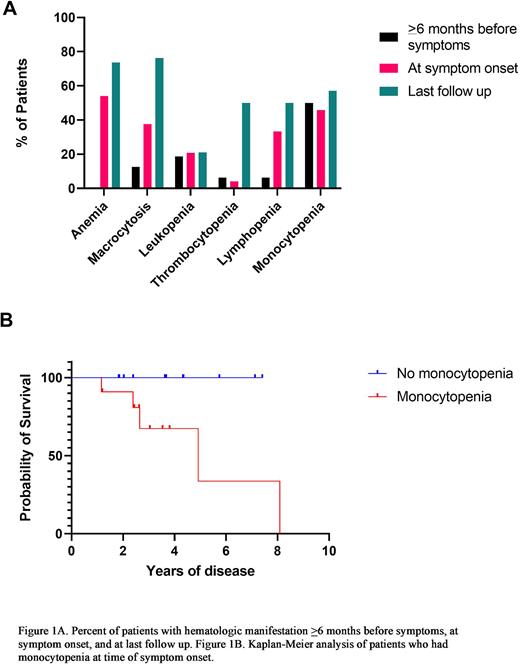
Prior to symptom onset (>6 months), laboratory data were available for 16 patients. At that time point, no patients were anemic, 13% (n=2) had macrocytosis, 19% (n=3) had leukopenia, 6% (n=1) had thrombocytopenia, 6% (n=1) had lymphopenia and 50% (n=8) had monocytopenia. Laboratory data was available for 23 of the 38 patients within 3 months of symptom presentation. At symptom presentation, most patients were anemic (n=13; 54%), 38% (n=9) had macrocytosis, 21% (n=5) had leukopenia, 4% (n=1) had thrombocytopenia, 33% (n=8) had lymphopenia and 46% (n=11) had monocytopenia. There were no significant differences in extent and duration of cytopenias between UBA1 mutational subgroups and in VEXAS patients with CH versus those without.
At last follow up (median 4.5 years, range 0.1-20.7), 74% (n=28) were anemic, 76% (n=29) had macrocytosis, 21% (n=8) had leukopenia, 50% (n=19) had thrombocytopenia, 50% (n=19) had lymphopenia and 58% (n=22) had monocytopenia. Nine patients (23%) were deceased due to infection (n=4), cardiovascular disease (n=1), stroke (n=1), and unknown cause (n=3). With median follow up of 4.5 years, patients with monocytopenia at the time of symptom onset had decreased median survival compared to those without monocytopenia (log-rank P=0.03). No other significant associations were identified.
Conclusions
We describe a timeline and spectrum of hematological changes in patients with VEXAS syndrome and demonstrate the adverse prognostic impact on survival associated with monocytopenia. Monocytopenia preceded symptom onset in 46% of patients, with the degree and duration of monocytopenia being independent of UBA1 mutational subtype and not being influenced by the presence or absence of clonal hematopoiesis.
Disclosures
Warrington:Eli Lilly: Other: Clinical Trial Support; GSK: Other: Clinical Trial Support; Kiniksa: Other: Clinical Trial Support; Chemocentryx: Consultancy. Patnaik:Kura Oncology, Stemline Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal